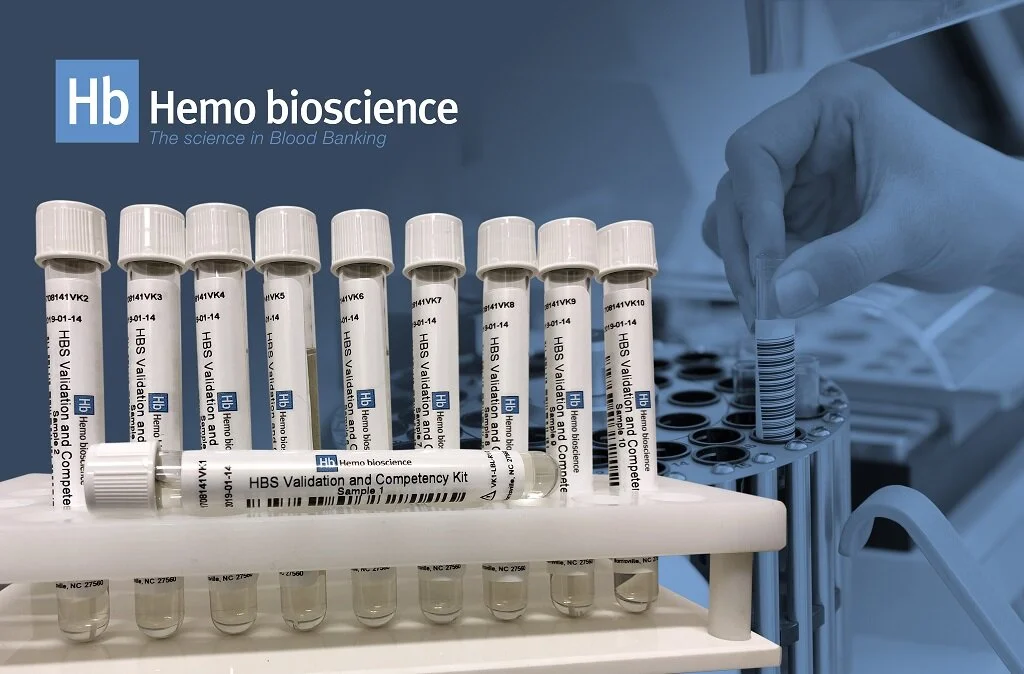
Blood Bank Validation - Validation and Competency Kit
Hemo bioscience has created a validation kit to be used for test system method validation and competency testing for testing technologists. The kit is meant to be used for manual, automated, and/or semi-automated reverse blood typing, antibody detection/identification, and cross-match compatibility testing systems. Our validation kit has been validated for testing with all methods. HB’s kit includes 8 positive samples, and 2 negative samples.
If you are interested in ordering our validation and competency kit, please contact us at (866) 332-2835 or email us at info@hemobioscience.com
Educational institutions qualify for a 15% discount.
| Product Name | Product Code | Package Size | Comments | Instructions | MSDS | Brochure |
|---|---|---|---|---|---|---|
Validation and Competency Kit |
H2010 |
10 x 5 mL |
Samples for Validation of automated and manual blood bank testing methods |
Comparison Chart
| Features | Immucor® Validation Panel | Quotient ALBAcheck® Comptency Testing Kit | Hemo bioscience™ Validation and Competency Kit |
|---|---|---|---|
| Ready to use for automation? | YES |
NO |
YES |
| IFU indicates suitability for testing on ALL available automated platforms? | NO |
NO |
YES |
| Intended for validation testing? | YES |
NO |
YES |
| Includes positive and negative samples? | NO |
YES |
YES |
| Guidance provided for cross-match compatibility testing validation? | NO |
NO |
YES |
| Sample Fill Volume? | 3 mL |
2 mL |
5 mL |
| Open/Closed Vial Stability? | 30 days/24 months |
24 months |
24 months |